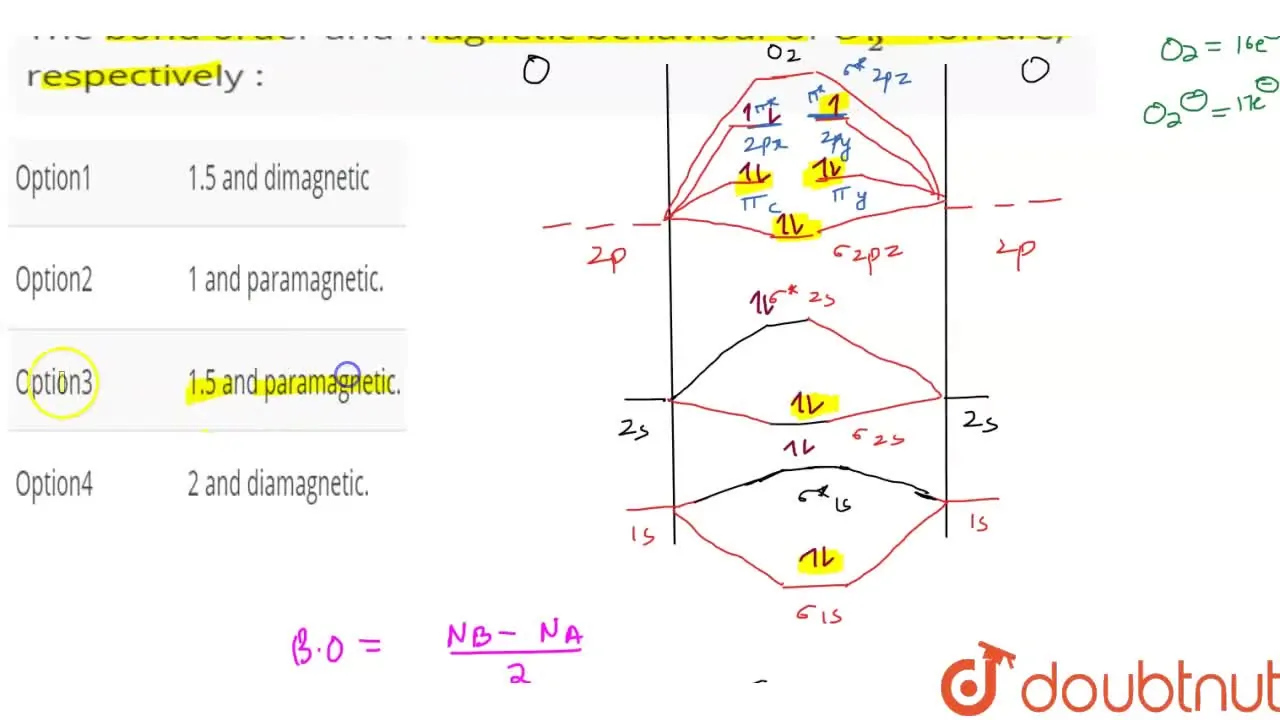
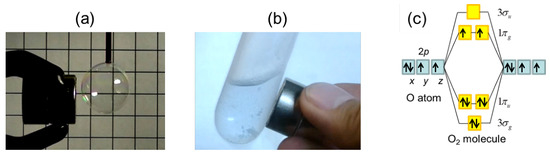
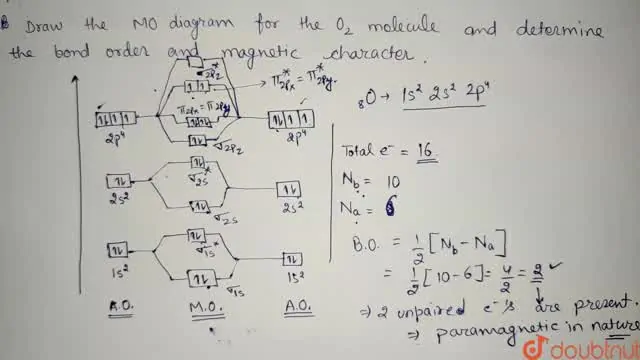
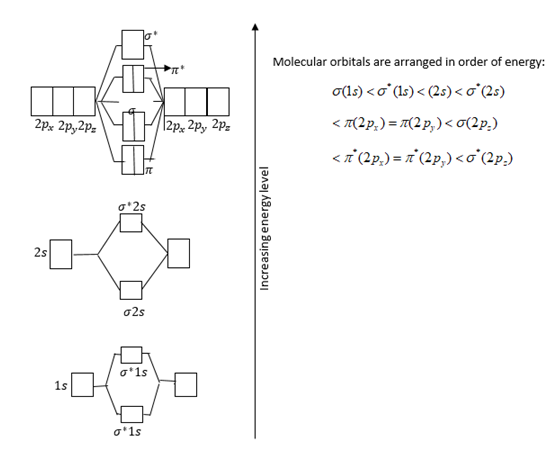
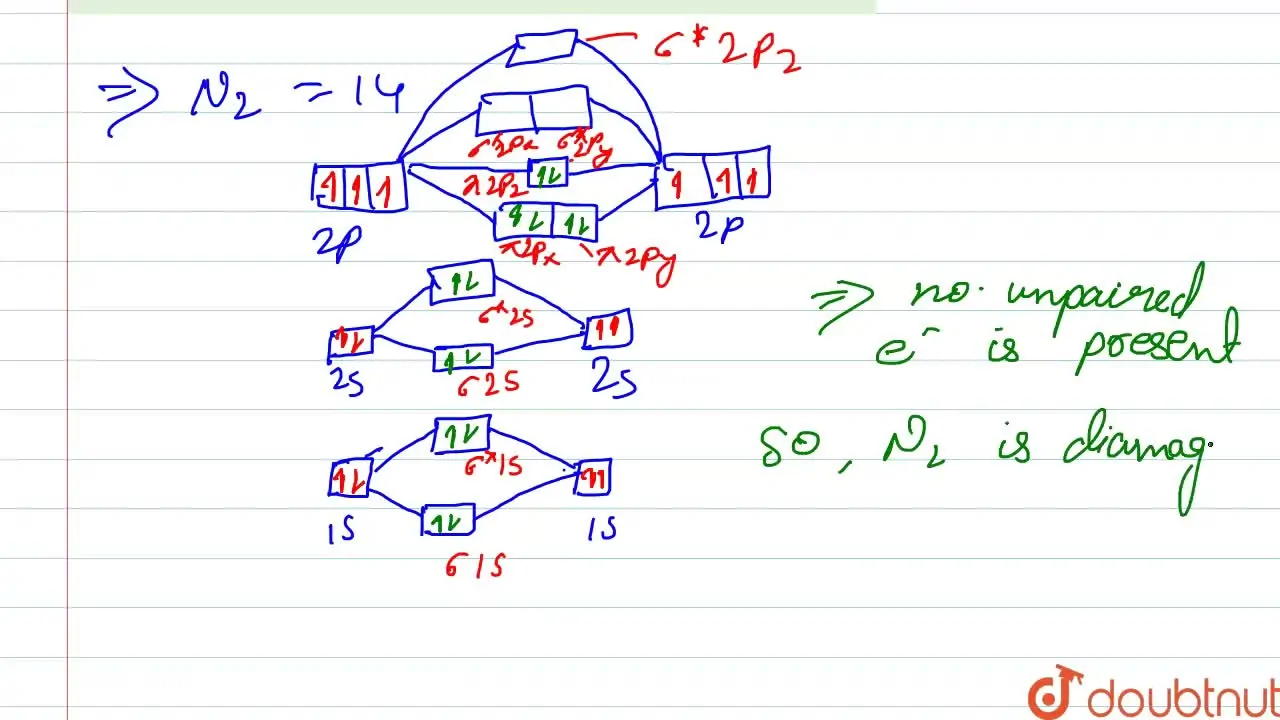
Write the molecular orbital electronic configuration of N2 and O2 molecules with the of molecular orbital theory. Predict its magnetic behaviour also.
Why is oxygen paramagnetic in nature and sulphur diamagnetic even though both belong to the same group? - Quora

How B2,O2, NO, NO2,ClO2 paramagnetic If I take B2 then it has 10 electrons so should be dimag - Chemistry - Chemical Bonding and Molecular Structure - 10867657 | Meritnation.com
57.: In which of the following ionisation processes, the bond order has increased and the magnetic behaviour has changed (a) NO to NO+ (b) O2 to O2+ (c) N2 to N2+ (d)
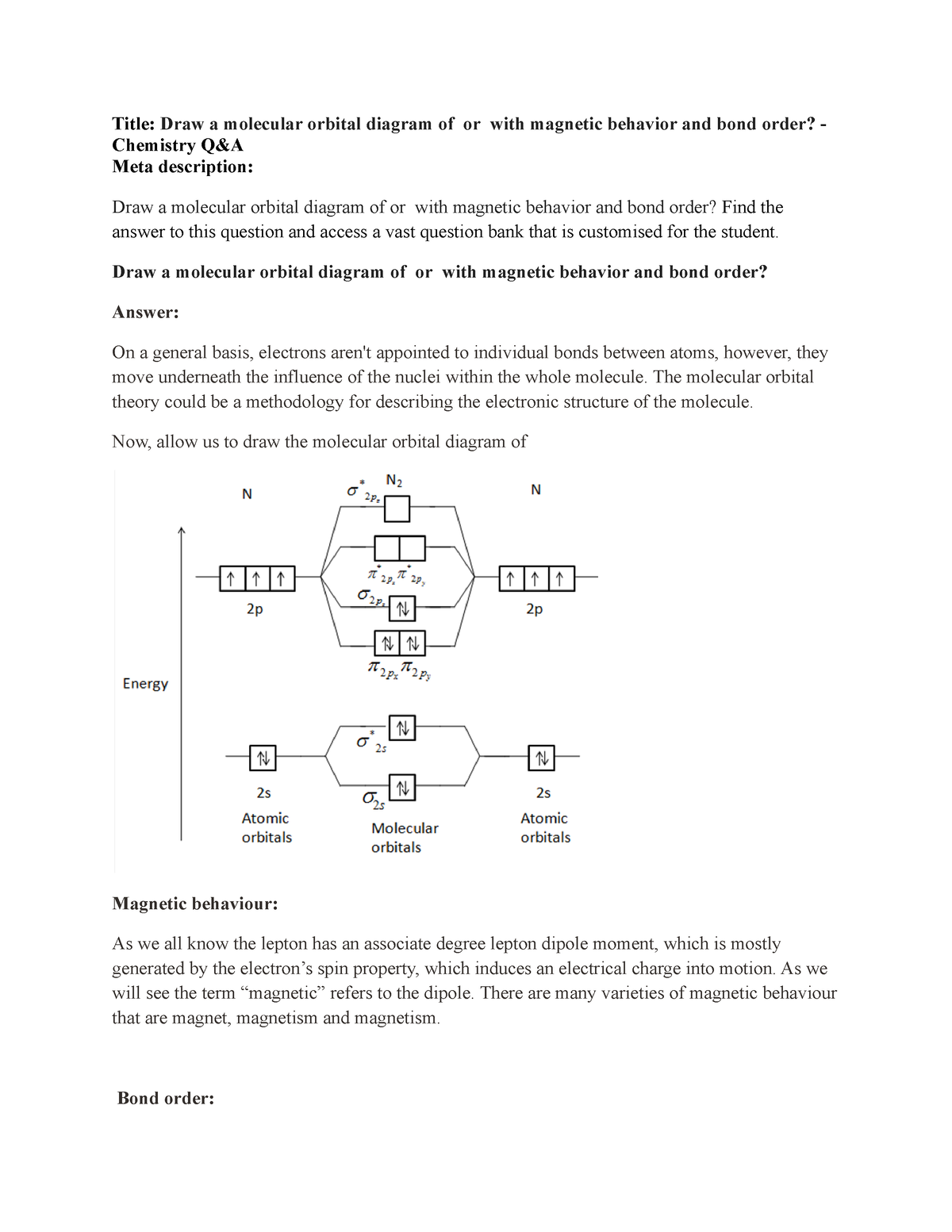
22-Draw a molecular orbital diagram of ${N 2}$ or ${O 2}$ with magnetic behavior and bond order - Studocu